 The growth of our Type 1 bacteria on YGC agar.
The growth of our Type 1 bacteria on YGC agar.| [Introduction] | [Methods] | [Discussion] | [Summary] | [References] |
| [Hoyer's Tubes] | [Colony Morphology] | [Wet Mount Motility] | [Gram Stain] | [Cell Shape] | [Endospore Stain] |
| [Catalase Test] | [Oxidase Test] | [Motility Stab] | [Fermentation Tube] | [Durham Tube] |
Summary
Two distinct organisms were isolated during this study. For the purposes of this discussion, the two organisms we isolated will be referred to as "Type 1" and "Type 2." Based on the outcomes of the various tests we performed, we can conclude that indeed, both types of bacteria we isolated were Acetobacter.
The Gram stain, endospore stain, catalase test, oxidase test, Hoyer's and Durham tubes gave us the necessary information to determine that our bacteria was Acetobacter. All of these tests on our bacteria gave the same results as what was delineated in Bergey's Manual. The bacteria were Gram negative and Gram variable, no endospores were present, catalase was present while oxidase was not, the bacteria was capable of metabolizing ethanol, and they were incapable of fermentation. The remainder of the tests helped us further describe and differentiate the bacteria.
| Sample | Results |
|---|---|
| Dirt A | some clouding |
| Dirt D | very little clouding |
| Apple Cider | little clouding in one, none in other |
| Co-op Apple | no clouding, just apple chunks |
| Fridge Apple | no clouding, just apple chunks |
| SKL | no growth |
As clouding indicates growth, we can conclude that bacteria grew from both soil samples and apple cider sample. Nothing grew from either apple sample or the Strawberry-Kiwi Lemonade. However, the bacteria that grew from these samples is not necessarily Acetobacter. Anything that can metabolize ethanol (the selective component of Hoyer's Media) will grow in the tubes, therefore tests must be performed on the organisms isolated from the broths to confirm whether or not they are Acetobacter.
NOTE: For the samples, Dirt A is the dirt sample we obtained from the Nyumburu Amphitheater and Dirt D is the sample from outside The Diner. SKL is Strawberry-Kiwi Lemonade. For further description of our samples, see the method section.
Type 1
 The growth of our Type 1 bacteria on YGC agar.
The growth of our Type 1 bacteria on YGC agar.
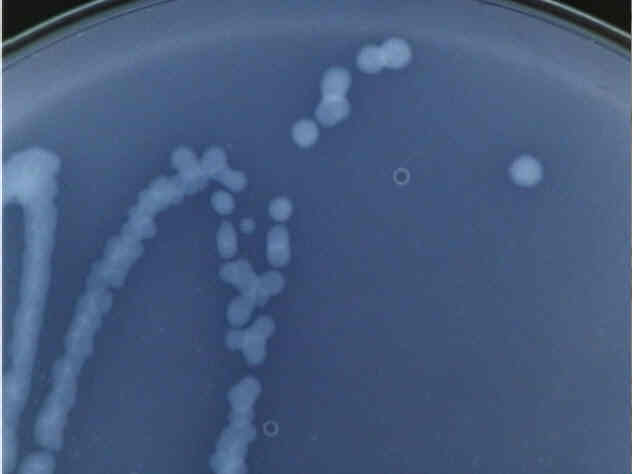 Close up of a Type 1 bacteria colony.
Close up of a Type 1 bacteria colony.
These colonies are white and translucent, rather difficult to see under normal lighting conditions, which is why the pictures were taken in such a way as to enhance contrast. Distinct colonies form, all fairly regular, medium-sized circles.
Type 2
 The growth of our Type 2 bacteria on YGC agar.
The growth of our Type 2 bacteria on YGC agar.
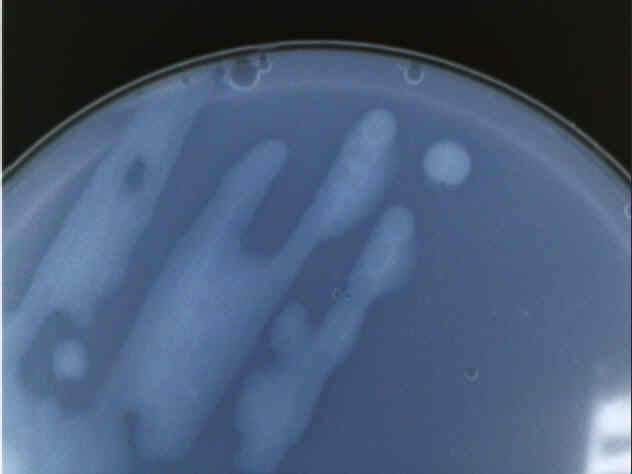 Close up of a Type 2 bacteria colony.
Close up of a Type 2 bacteria colony.
Perhaps the most distinctive feature of our Type 2 bacteria is its reluctance to form distinct colonies, as can be seen in both the picture of the entire plate and the detail of only two distinct colonies that formed. Also, the colonies were not very cohesive; when turned upside-down, the bacteria had a propensity to drip onto the lid of the petri dish. Other than that, Type 2 was also translucent and appeared to lack any pigment other than a faint yellow color.
Both Type 1 and Type 2 bacteria showed definite taxis, often tumbling and changing direction. It is likely that both bacteria have flagella, although without an electron microscope, we are unable to determine the number or arrangement of the flagella. As Acetobacter can be either motile or non-motile, this test does not allow us to conclusively name our bacteria, although it does help us to be able to further describe them.
| Type | Result |
|---|---|
| 1 | purple rods |
| 2 | pink rods |
| Type | Result |
|---|---|
| 1 | pink rods |
| 2 | pink rods |
The Type 2 bacteria proved to be Gram negative, in agreement with the Bergey's description that Acetobacter is Gram negative. On the other hand, Type 1 at first stained purple, meaning it was Gram positive. As some Acetobacter will be Gram variable, we decided to stay on the safe side and re-stain both bacteria types. Type 2 stayed Gram negative and Type 1 stained Gram negative, indicating that it is a Gram variable strain. Both these results further correspond with Acetobacter.
NOTE: If you would like to see what the cells would have looked like in their Gram negative state, check out the endospore stain.
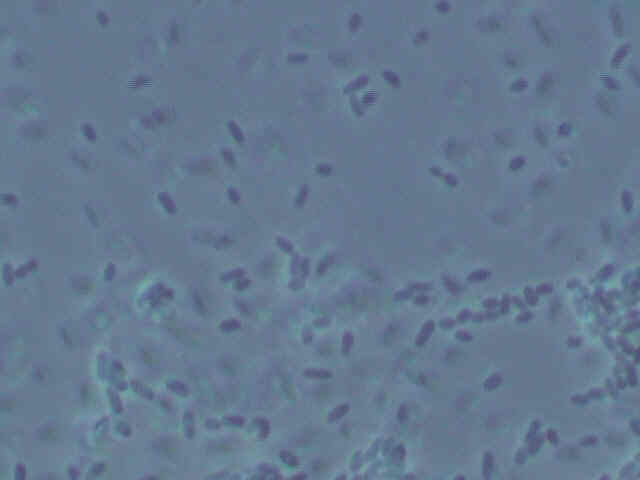 Picture of Type 1
bacteria.
Picture of Type 1
bacteria.
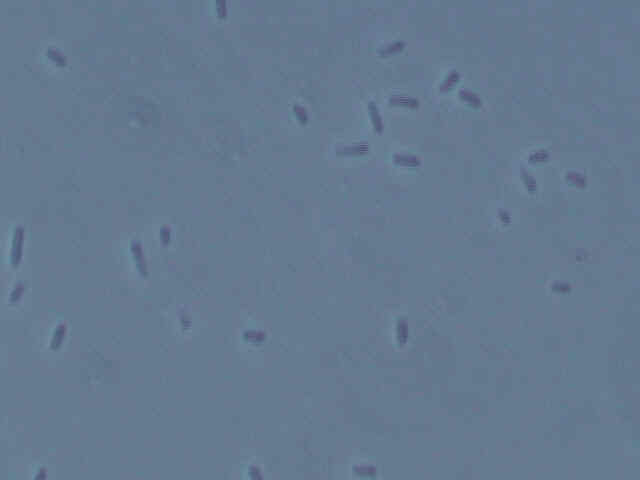 Picture of Type 2 bacteria.
Picture of Type 2 bacteria.
Both types of bacteria are small, squat bacilli with a bit of a roundish appearance to them. Some types of Acetobacter also are small, roundish bacilli. However, as many types of bacteria look similar in shape, this is no definitive observation. More tests are in order to determine the identity of the bacteria.
| Type | Result |
|---|---|
| 1 |  |
| 2 | 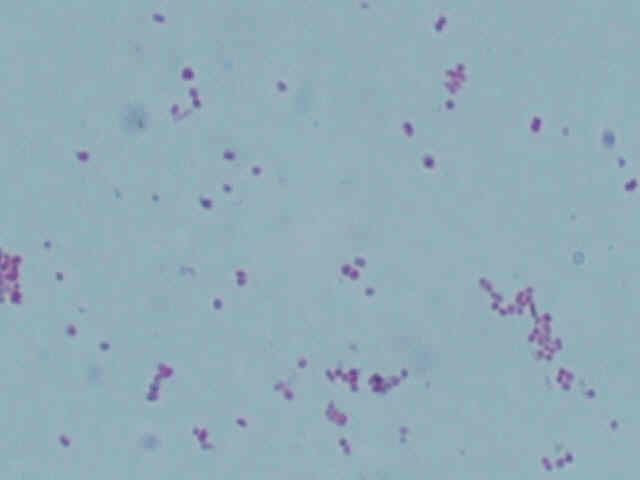 |
As can be seen by the lack of green dots in the center of the pink vegetative cells, neither type of bacteria form endospores. This corresponds with Acetobacter, which also does not form endospores.
| Type | Result |
|---|---|
| 1 | no change |
| 2 | no change |
After placing the oxidase testing liquid over the bacteria on the swab, no blue color developed, which would have indicated a positive test for oxidase. As Acetobacter is listed as not containing oxidase in Bergey's Manual, this test further proves the bacteria's identities as Acetobacter.
| Type | Result |
|---|---|
| 1 | bubbles |
| 2 | bubbles |
As soon as hydrogen peroxide was placed on the sample of the bacteria, oxygen bubbles immediately evolved, signifying a positive reaction and that the catalase enzyme is indeed present in both types of bacteria. This corresponds with the description of Acetobacter.
Both Type 1 and Type 2 bacteria are motile. Acetobacter can be either motile or non-motile, so although this test does not get us any further to proving or disproving the bacteria's identity, it does give us more information on the bacteria itself.
| Type | Oxidative | Fermentative |
|---|---|---|
| 1 | yellow, gas in tube | yellow, gas in tube |
| 2 | yellow, gas in tube | yellow, gas in tube |
Due to the color change and production of gas in both tubes, we should assume that fermentation took place, especially considering that we modified this test slightly and cut off the air supply to the broth culture by covering the top with a layer of mineral oil. Judging by the color change, acid was produced, and according to the bubble trapped in the glass tube, gas was also produced. This should not have happened with Acetobacter as they are supposed to be obligate aerobes, and growth in the absence of oxygen clearly does not support the assumption that these bacteria are Acetobacter.
However, upon further consideration, we decided that these results were invalid and do not contribute in any way to the identification of our bacteria. This is because this test fails to take into account that there are other things besides oxygen that act as the final electron acceptor in the electron transport chain. All this test proves is that an acidic compound is produced by the bacteria and that gas is evolved, both which Acetobacter do, but not very helpful in proving one way or another what our samples are.
| Type | Results |
|---|---|
| 1 |  |
| 2 |  |
At first glance, it appears that the bacteria were able to ferment as the tubes have all been sealed off by mineral oil and a majority of the tubes have turned yellow. However, to have a positive test for fermentation in the Oxidative/Fermentative Durham tubes, the tube needs to have completely turned yellow. As the bottoms of all the tubes are still some shade of green, this is a negative test for fermentation, and both types of bacteria are obligate aerobes. Acetobacter is also obligatory aerobic, therefore this test helps us prove that the bacteria we isolated are Acetobacter.
The reason for the misleading results is probably due to oxygen getting trapped in the media well before being sealed off by the mineral oil. This hypothesis is supported by the fact that there was only growth in the upper portion of each tube, and the bottoms remained green. As oxygen is a gas, it would diffuse up, but then it would be trapped by the layer of mineral oil, thus creating a greater concentration of oxygen around the top of the media. This allows oxidation to occur, even in what should be an anaerobic environment, and this is likely what occurred in our tubes.

In addition to the two types of bacteria we isolated, we also managed to grow yeast in Hoyer's media. Evidently, this organism is also capable of metabolizing ethanol.
An interesting side note about this yeast is that there appears to be some other organism inside of it. If you look at the picture carefully, you will notice that in addition to the large mass of the nucleus, there is often a smaller, oblong body in the cell. What cannot be shown in a still photograph is the interesting and directed movement this small thing displayed. We are not sure if this intracellular object is a parasitic or symbiotic organism or if it is just a visible side-effect of the cell's metabolism. Although not an intended part of our project, we found it interesting and deemed it worthy of inclusion.