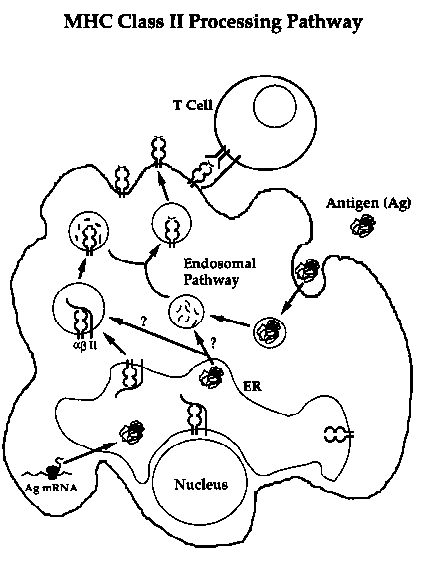

My specific project was the investigation of the role of the Invariant Chain protein in the Class II MHC pathway. The invariant chain (or Ii for short) is a Type II (N-terminus in the cytoplasm, C-terminus in the lumen) membrane glycoprotein which associates with newly synthesized Class II MHC molecules in the ER. Although there are multiple forms of the invariant chain (due to alternative transcription and translation initiation start sites, alternative RNA splicing, and multiple sites of post-translational modification), the most abundant is the p31 form, and this is the form I studied.
Although there are several proposed roles for the invariant chain, its mechanism(s) of action are poorly understood. It has been shown to target MHC molecules to an endosomal compartment, where antigen processing is thought to occur, and it is believed to act as a chaperone in the proper folding of Class II molecules in the ER. There is also evidence that it may exclude ER-resident peptides (such as those transported by the TAP complex) from the Class II molecules. In addition, some recent studies suggest that the invariant chain may act as a selective protease inhibitor, perhaps indicating that it also plays a more direct role in the generation of antigenic peptides.
We found that the invariant chain can influence the processing and presentation of both endogenously-expressed and exogenously-added antigens. COS (and more recently, the human line 293.T) cells transfected with the murine A(k) Class II molecule can process exogenous ovalbumin and present the OVA(247-265) epitope to specific T cells, but they do so poorly unless co-transfected with the murine p31 Ii. Further, they can only present the OVA(247-265) epitope from endogenously expressed ovalbumin if they co-express the invariant chain. Intriguingly, the opposite is true for the hen-egg lysozyme epitope HEL(34-45). Presentation of this peptide from endogenously expressed HEL is inhibited by co-expression of the invariant chain, although presentation of the same peptide from exogenously added HEL is improved in the presence of Ii. Thus, while the invariant chain appears to generally improve the presentation of exogenous protein antigens, it has different effects on the presentation of endogenous antigens.
In order to get at the mechanisms of these effects, I examined the functionality of a number of deletion and substitution mutants of the p31 Ii. For "functionality", I examined two basic criteria: association with Class II MHC molecules (A(k) specifically) and effects on the presentation of the abovementioned OVA and HEL epitopes. I identified several regions of functional importance, although no mutations tested destroyed A(k)-association. These regions are:
Unlike with OVA, presentation of endogenously expressed HEL (HEL34-45
epitope) is inhibited by co-expression of Ii, indicating that processing
from intracellular HEL is different from processing of extracellular
protein. Somewhat surprisingly, we found that presentation of HEL34-45
from endogenous HEL was not inhibited by treatment with
chloroquine, although processing of extracellular HEL was completely
blocked by similar treatment. Further, targeting HEL to the endosomes by
fusion with the Ii endosomal targeting signal restored sensitivity to
chloroquine, strongly suggesting that endogenous HEL is processed in a
non-endosomal compartment. This would also explain inhibition by
wild-type Ii (which redirects Class II molecules to the endosomes) but
not a targeting mutant of Ii. Additionally, we found that presentation of a
second epitope, HEL74-88 (on the A(b) Class II molecule) follows the same
pattern as HEL34-45. Thus, endogenous proteins can be processed in
different pathways within the same cell, which may have implications for
T cell repertoire selection and reactivity in the periphery.
Back to Ken's Home Page