Table of Contents
Abstract
Introduction
The Clientele
Course Content
1. Chain of Custody
2. Drug Analysis
3. Toxicology
4. Trace Analysis/Arson
5. Serology/DNA Analysis
Conclusion
References
"Dr. Watson, Mr. Sherlock Holmes," said Stamford, introducing us.
Analytical chemistry is the science that addresses methods used to determine the quantitative or qualitative composition of unknown samples. Although frequently neglected, the nature of the sample and the use of the analytical chemical information play important roles in selecting and executing the appropriate chemical analysis technique. In few areas is this more obvious than in forensic chemistry. Forensic chemistry is a timely subject that serves as a palette from which we can stimulate the interests and abilities of our students. With the backdrop of forensic decision-making, the real world relevance of chemical measurements is obvious to students.
In the spring of 1997, the authors collaborated to teach a senior-level elective course covering analytical chemical methods in forensic science including gathering of evidence, drug identification, toxicology, trace evidence, arson, and DNA/serology. The textbook used for the course (Saferstein, R. Criminalistics; An Introduction to Forensic Science, 5th Ed., Prentice-Hall, Inc., NY, 1994) was supplemented by handouts based on the forensic and analytical chemistry literature.
In this article, we discuss selected aspects of the course, focussing on topics not generally covered extensively in instrumental analysis courses.
Prerequisites for the course were set loosely, hoping to attract at least a dozen students. The course prerequisites were: one year of general chemistry, one year of organic chemistry, and one semester of quantitative analysis. We recommended, but did not require, students to have completed one year of general biology The course was approved at a late date, the course listing did not appear in the University course schedule, and the course was advertised primarily by flyers in the elevator lobby. Over forty students including three quarters of the senior chemistry majors, a good complement of students from Biology, and students from Pharmacy enrolled for credit. We even had two students visiting from other educational institutions attend the first few weeks until their programs started.
An outline of the course content is shown in Table 1. The broad scope
of the course necessitated a team teaching approach. Invited speakers and
a mock trial brought forensic examples from actual case studies into the
classroom. The following sections highlight some of the more unusual aspects
of the course.
Click here
to view Table 1. Lecture topics
1. Chain of Custody
The single most important aspect of all forensic chemistry is maintaining the chain of custody. Chain of custody refers to the time course in which evidence was handled and includes every person who handled the evidence. It is imperative that evidence be handled by the minimum number of people needed to complete the forensic analysis. An ideal chain of custody is one that involves two individuals, one person who collects and delivers the evidence, and one person who analyzes the evidence. All persons in the chain of custody must be prepared to testify in court to validate the integrity of the evidence. If the chain of custody is broken in any way, the evidence will be excluded from court testimony, which often results in the dropping of criminal charges against the defendant.
Chain of custody arguments were key factors used by the defense in the O.J. Simpson trial. Because the prosecution was unable to account for all the blood taken from the suspect, the defense was able to present the possibility of evidence tampering.
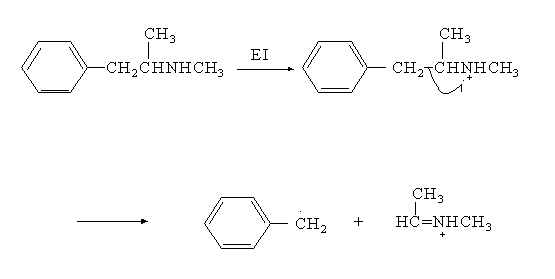
Forensic drug analysis deals with the identification of illicit (illegal) drugs. Because of the increased popularity of "crack" (free base) cocaine, the majority of drug analysis cases involve cocaine analysis. Drug classifications by pharmacological similarity and by U.S. federal law were discussed in this course. Spot tests for the analysis of opiates (such as heroin and morphine), amphetamines, barbiturates, marijuana, lysergic acid diethylamide (LSD), and cocaine were described. Methods of analysis covered include UV/Vis, FTIR, and GC/MS. GC/MS is most often used for drug identification, and topics in mass spectrometry, such as mechanisms of fragmentation, were covered in great detail. For example, in interpreting the mass spectrum of methamphetamine (speed or crank) a base peak of 58 amu is found. The mechanism for the formation of this fragment is illustrated in Figure 1.

Forensic toxicology deals with qualitative and quantitative analysis of biological specimens for the presence of alcohol, drugs, and/or poisons and their corresponding metabolites. Investigations often involve driving-under-the-influence (DUI) of alcohol or drugs or death investigation. In death investigation, the role of the toxicologist is to assist the coroners and pathologists in determining the cause and manner of death (natural, accidental, suicide, or homicide).
The class first covered lectures on alcohol, one of the most abused drugs in the U.S. The theory of breath testing and instrumentation such as the Breathalyzer (and DataMaster) were described. The course then covered the pharmacology of ethanol, the Widmark equation, and the analysis of ethanol in blood and other biological fluids and tissues. A mock court was used in class to exemplify the relevance of courtroom testimony. A student volunteer played the role as an expert witness in forensic toxicology for a DUI case. The student had to answer questions involving chain of custody, blood alcohol analysis, QA/QC, and the interpretation of the results pertaining to possible impairment.
The course subsequently covered the analysis of numerous drugs and metabolites. Methods of analysis presented in class included GC, immunoassays, TLC, GC/MS, HPLC, and HPLC/MS. Drug extraction methods by liquid phase and solid phase techniques were also described.
Click here for sample homework problem 2
Click here for sample
homework problem 3
The basis for trace analysis in forensic science is the statement made in 1910 by the French criminologist, Dr. Edmond Locard: "Every contact leaves a trace." Since physical contact is involved in almost every crime, the analysis of trace evidence plays a crucial role in crime scene investigation. The trace applications in forensic chemistry included the analysis of gun shot and primer residue, paint, hair, and fibers.
To determine whether a suspect fired a weapon, a sample is obtained by swabbing the back of the hand with dilute nitric acid or pressing adhesive stamps on the back of the hand. The swabs or adhesive stamps are subsequently analyzed by AA, ICP/MS, and/or SEM. Also, the distance at which a weapon was fired can be approximately determined by comparing patterns of gun shot residue at various distances. Paint analysis is especially useful in hit-and-run cases, where the paint chips are analyzed by pyrolysis GC, FTIR, and SEM. The first two methods are used for comparing the organic binder in the paint with that of a standard (for example, paint from a suspect's vehicle). SEM is used for identifying the constituents of the inorganic pigments present in the paint. Hair and fiber analysis is usually performed by a comparison microscope where the known standard is physically compared to the evidence. A polarizing microscope is often used to identify the class of fiber.
Forensic arson analysis deals with the analysis of fire debris for the presence of accelerants. Some common accelerants found in arson cases are gasoline, kerosene, and charcoal lighter fluid. Fire debris submitted for arson analysis are packaged in sealed containers such as mason jars or empty paint cans. The chemist performs the analysis by inserting activated charcoal (C-strips) into the "headspace" of the sealed container. The C-strip is subsequently removed from the container and placed in carbon disulfide. The accelerants desorb from the C-strip and dissolve in the solvent, then the solution is injected into a GC or GC/MS. The identification of hydrocarbon constituents is performed by comparison to known standards.
A new method of arson analysis involving solid-phase microextraction (SPME) followed by gas chromatographic analysis was also discussed. Volatile molecules in the headspace of a sample sorb to a silica fiber coated with a polymeric stationary phase. These components are subsequently thermally desorbed in the injection port of the GC and analyzed by GC or GC/MS. A gas chromatogram of a charcoal lighter fluid standard obtained by SPME is shown below.

Figure 2. A gas chromatogram of a charcoal lighter fluid standard that was extracted by SPME.
Click here for sample
homework problem 4
Forensic serology deals with the study of body fluids that might have probative value in the prosecution of a crime. Typical fluids are blood, semen, and saliva. After testing to identify the substance, it is possible to begin to identify the donor of the material by testing for "blood group substances" (from the ABO blood group system) or other proteins that are polymorphic. In this portion of the class, students became familiar with such biochemical techniques as immunoelectrophoresis, isoelectric focusing, and ELISA. Due to the discrimination power of DNA technology, several basic serology tests have been discontinued in the past decade. However, it is still imperative to identify the origin of a body fluid before results from its analysis are presented in court.
Forensic DNA analysis deals with the identification of the source of a body fluid through DNA testing. Since its inception more than a decade ago, it was anticipated that forensic scientists would be able to identify the source of biological evidence with the same certainty that traditional fingerprint evidence has always been presented in court. With more loci available for testing, the FBI recently declared that it would now report that "John Doe is the origin of the blood found at the crime scene, within a degree of scientific certainty." DNA testing falls into two categories: RFLP (restriction fragment length polymorphism) via Southern Blotting and hybridization with labeled DNA, and PCR (polymerase chain reaction) followed by a variety of detection methods. Students learned the advantages and sample requirements for each technique. The PCR-based tests introduce a variety of analytical procedures including capillary electrophoresis, fluorescent imaging, and MALDI-TOF (matrix-assisted laser desorption ionization) mass spectrometry.
Click here for sample homework problem 5
Click here for sample
homework problem 6
We will teach this course again. The course was the second highest
rated course in the Department of Chemistry and Biochemistry for Spring
1997. The overall course evaluation score was 3.68/4.00 (with a standard
deviation of 0.53 for 34 respondents). The average in the Department
was 2.76 with a standard deviation of 1.06 for 1127 responses. A
numerical summary of student responses follows.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Several "outcome" questions were also asked; the results follow.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The following statistics reveal the attractive nature of a course in
forensic analytical chemistry. Relatively few students took this
course to fulfill degree requirements.
|
|
|
|
|
|
|
|
|
|
|
|
Click here to see some representative comments from students in the course.
One student suggested that the course could have been better titled as “Instrumental analysis with forensic applications.” Indeed, teaching real-world applications alongside our traditional presentations of instrumental analysis is an excellent way to motivate students. Even though analytical chemistry is an applied science, our undergraduate curriculum emphasizes theory and instrumentation often at the expense of practical aspects of chemical analysis. This forensic analytical chemistry course served to correct for a curriculum that leaves students clueless about when and how analytical methods are practically employed. We will emphasize practical examples of chemical analysis in other courses that we teach.
The outcome is not just good course evaluations; the outcome is students
who are more knowledgeable about the everyday use of analytical chemical
methods.
Baden, M. M.; Hennessee, J. A. Unnatural Death: Confessions of a Medical Examiner, Ballantine Books, New York, 1989.
Brewer, W. E.; Galipo, R. C.; Morgan, S. L.; Habben, K. H. “Confirmation of volatiles by solid-phase microextraction and GC/MS,” J. Anal. Toxicol. 1997, 21(4), 286-290.
Davis, G. Forensic Science, American Chemical Society, Washington, DC, 1986.
Dwyer, J.; Kocieniewski, D.; Murphy, D; Tyre, P. Two Seconds Under the World: Terror Comes to America (The Conspiracy behind the World Trade Center Bombing, Crown Publishers, Inc., New York, 1994.
Evans, C. The Casebook of Forensic Detection, John Wiley & Sons, New York, 1996.
Fisher, D. Hard Evidence, Bantam DoubleDay Dell Publ. Group, NY, 1995.
Gerber, S. M., Ed., Chemistry and Crime; From Sherlock Holmes to Today's Courtroom, American Chemical Society, Washington, DC, 1984.
Ho, M. H. Analytical Methods in Forensic Chemistry, Ellis Horwood, Ltd., London, 1990.
Hunt, S. M. Investigation of Serological Evidence: A Manual for Field Investigators, Charles C. Thomas Publ. Ltd., London, 1984.
James, R. E. Laboratory Manual for Criminalistics, Prentice Hall, NY, 1980.
Lowry, W. T. Forensic Toxicology: Controlled Substances and Dangerous Drugs, Plenum Publ. Co., NY, 1979.
Maples, W. R.; Browning, M. Dead Men Do Tell Tales, Bantam DoubleDay, NY, 1994.
Saferstein, R. Criminalistics; An Introduction to Forensic Science, 5th Ed., Prentice-Hall, Inc., NY, 1994.
Saferstein, R. Forensic Science Handbook, Vol. I-III, Regents/Prentice Hall, NJ, 1993.
Tebbett, I., Ed., Gas Chromatography in Forensic Science, Ellis Horwood, Ltd., London, 1993.
Terry, I. M.; Robertson, J. C. Instrumental Data for Drug Analysis, CRC Press, Boca Raton, FL, 1991.
Wecht, C.; Curriden, M.; Wecht, B. Grave Secrets, Penguin books USA, Inc., New York, 1996.
Widmark, E. M. P. Principles and Applications of Medico-Legal Alcohol Determination, translated from original 1932 ed., Biomedical Publications, Davis, CA, 1981.
Yinon, J., Ed., Forensic Applications of Mass Spectrometry (Modern Mass Spectrometry), CRC Press, Boca Raton, FL, 1995.