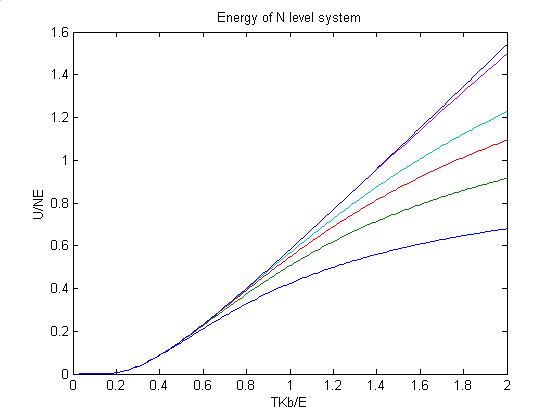
In the class of ENMA461 we have discussed the system with two level of energy(Fermi-Dirac system) and the system with unlimited energy per atom( Einstein system). However, what will happen if a every atom in a system could stay in n levels of energy? Here I calculated this kind of system and show the result here.
The first picture shows the energy of n-level system. From the lower to higher the number of levels are 3, 4, 5, 10, 30. From this piture we can see that as the number of levels goes up, the energy of system looks more and more like Einstein system below certain temperature. And if the melting point reaches before the n-level crystal could reach the maxima point of its energy, we can say that it display properties of Einstein crystal. The y axis is U/(nŽ┼). And x axis is TKb/Te.
The second picture shows the energy of crystal with more levels. In this picture, we can affirm that if the n-level crystal has enough energy levels, it will show properties of einstein crystal before it reaches its melting temperature.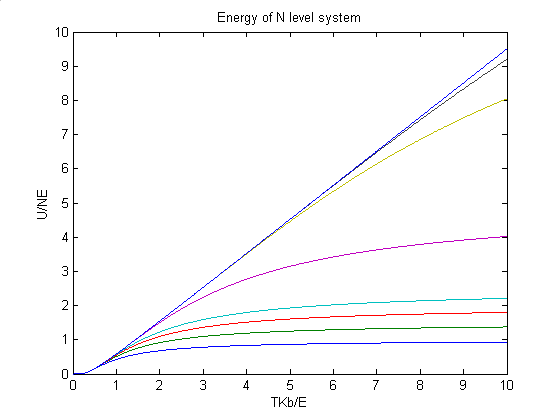
The third picture shows the heat capacity of a n-level system. In this picture, the heat capacity curves confirm our conclusion again, that is, if the number of levels in a n-level crystal is large enough, the crystal acts as if it is an Einstein crystal.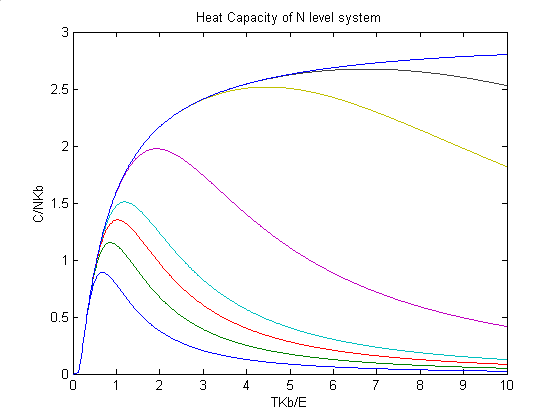
Nonetheless, I have to mention that the n-level system still has a heat capacity curve profile like 2-level system, which could be show in the illustration below. But the key is, before it could exhibit that kind of profile, the crystal may reaches its melting point, as a result, what we can see is only portion of the curve and almost the same as Einstein system.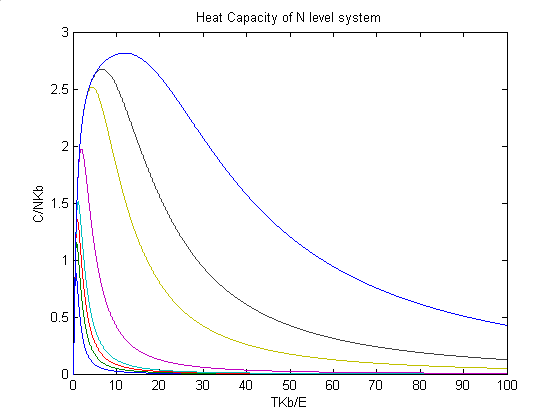
Below certain temperature, if the number of levels in a n-level system is large enough, the n-level system will display properties of Einstein system.