Lab 11 Immunoprecipitation
In this lab exercise, we will use the specific binding affinity of antibodies to antigens to purify antigens. We are going to purify bovine serum albumin (BSA) from fetal bovine serum (FBS).
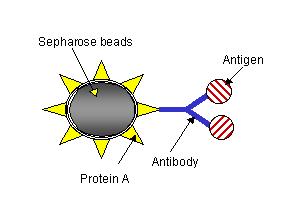
To purify BSA from FBS, anti-BSA antibody will be used. Anti-BSA antibody will bind to protein A conjugated to agarose gel. Protein A and protein G are proteins from the cell wall of the bacteria Staphylococcus aureus and Streptococcus, respectively. One of the important characteristics of these proteins is their high affinity for the Fc portion of antibody molecules from several animal species. Although no biological explanation for the high affinity of these proteins for antibody has ever been discovered, they have become very useful reagents in immunoprecipitation and affinity chromatography. Protein A is covalently conjugated to agarose gel (protein A-agarose gel). The Fc portion of the antibody binds to protein A, and the antigen binding sites of the antibody are exposed to bind to antigens. The agarose gel increases the size of antibody-antigen complexes so that they can be easily spun down. Antigen and antibody can be released from protein A-agarose gel by detergents or a low pH solution. When a small amount of antigen-bound protein A-agarose gel is used, only a small amount of antigen can be purified, which is called immunoprecipitation. When antibody-bound protein A-agarose gel is packed in a column, a great amount of antigen can be purified, which is called affinity chromatograph. Alternatively, antibody directly conjugated to agarose gel can be used to substitute antibody-bound protein A-agarose gel.
The following lists a few of the applications of the immunoprecipitation and affinity chromatography.
1. Protein A-agarose gel is commonly used to purify IgG antibodies from sera.
2. Antigens conjugated to agarose gel are used to purify antibodies specific for the antigens.
3. Antibody-bound protein A-agarose gel or antibodies directly conjugated to agarose gel are used to purify antigens.
March 11 Setting up the immunoprecipitation
1. Each group will have two students. You have two eppendorf tubes containing 0.5 ml FBS. To each tube, add 0.5 ml 1%NP-40 (a detergent) lysis buffer (final concentration: 1% Nonidet P-40, 50 mM Tris/HCL, pH 7.4, 150 mM NaCl, 5 mM EDTA, and proteinase inhibitors).
2. Vortex the samples 3 times during 5 min.
3. Spin the samples in a microcentrifuge for 30 min at 4oC to remove debris.
4. Label two eppendorf tubes as A and B. Add the antibody and protein A Sepharose beads as described in the table.
Tube A Tube B Anti-BSA antibody 5 ml - Protein A gel 25 ml 25 ml Please resuspend the protein A beads very well before you use it, and use a pipetteman (p200) and a cut tip to add protein A beads.
5. Transfer the supernatant of FBS lysate to the tube A and B, and discard the pellet.
6. Rotate the samples for 48 h at 4oC.
March 13 Wash immunoprecipitates
7. Spin the samples in a microcentrifuge at a full speed for 10 sec. Remove and discard the supernatant with a p1000 pipetteman. Leave ~100 ml of the supernatant in the tube and be very careful not to disturb the pellet.
8. Wash the beads with 0.5% NP-40 wash buffer (the same as the lysis buffer except NP-40 is 0.5%) for 3 times. To wash, add 1 ml 0.5% NP-40 wash buffer to each sample. Invert the tube 4 times to resuspend the beads. Spin the sample at the full speed for 10 sec. Remove and discard the supernatant and leave ~100 ml supernatant in the tubes. Again please be very careful not to disturb the bead pellet. Repeat it for two more times.
9. Wash the beads with PBS once as described at 8 to remove the detergent.
10. Your TA will store your samples at –20oC for further analysis.
Study Questions
1. Is the affinity-purified antibody from the protein A gel pure with respect to antigen specificity? Why or why not.
2. How could you use immunoprecipitation to purify CD3 from T cells.
3. In the lab exercise of immunoprecipitation, we used a BSA-specific antibody and protein A-Sepharose beads to isolate BSA from bovine serum.
a. Please explain the purpose protein A-Sepharose beads serve.
b. If we only add the BSA-specific antibody but not protein A-Sepharose beads, will we be able to isolate BSA from bovine serum, and why and how?
c. If we add F(ab’)2 fragment of the anti-BSA antibody instead of the intact antibody with protein A –Sepharose beads, what will happen and why?
d. If we add Fc fragment of the anti-BSA antibody instead of the intact antibody with protein A –Sepharose beads, what will happen and why?