Chem 122 Laboratory, Spring 1996
RETURN to index
Chemistry Software Tools
This activity must be done in the Chemistry Macintosh Room, Chem 2305, just one
flight up from our laboratory. (The WAM labs don't have the required software).
This computer room is a public facility that you can use anytime
during its open hours (9 am - 5 pm weekdays). It has word processors and other
common software but not the same software that the WAM labs have.
1. Dot structures in Beaker
Find the Beaker icon and double-click on it. Click on the opening
screen to clear it, then pull down the Struct menu and select Lewis
Dot Diagrams. Beaker knows the electron configuration of the elements up
to Ne and will attempt to draw the "Lewis dot structures" of simple formulas
you type in the box at the bottom of the window. In drawing these structures,
the computer will use the correct total number of valence electrons for all
atoms and will attempt to "obey the octet rule" insofar as possible (it's not
always possible, as you will see).
a. What is the "octet rule"?
Referring to your periodic table, type in the element symbols for each element
from H to Ne and press return after each one. The computer draws the element
symbol with dots around it representing the valence shell electrons.
b. Try typing the formulas for O2, N2, Cl2,
H2O, H2O2 (hydrogen peroxide, used
in bleaching hair), CH4 (methane), NH3 (ammonia),
C2H6 (ethane). Do these dot
structures obey the "octet rule" if possible?
c. Now try to make the series CH, CH2, CH3, CH4, CH5. Which is the only one
that the program will draw that obeys the octet rule?
Why won't it draw a dot structure for CH5?
d. Use the program to predict the likely structure of a compound of
boron and chlorine. Is it possible for this compound to obey the
octet rule? Why not?
e. What dot structure does the program draw for carbon monoxide. Does
it obey the octet rule? Why do you think the program puts -1 and +1 signs on
the structure? (Hint: compare this dot structure with that for N2, which has
the same number of total valence electrons, 10, and the same dot structure, yet
does not have the -1 and +1 signs).
f. What dot structures does the program draw for ozone, carbon
dioxide and nitrous oxide N2O? What does the double line represent?
(Don't worry about the resonance structures for the time being).
g. What dot structures does the program draw for nitrogen oxide (NO) and
nitrogen dioxide (NO2). Is it possible for these compounds to obey the octet
rule? Why not?
h. Have the program draw the series C2Hn, where n = 1 through 7
Which are the only ones that obey the octet rule?
2. Drawing and Naming Organic Compounds in Beaker
a. The rules for the structure and naming of organic compounds (compounds of
carbon) are so systematic and predictable that it is possible to program a
computer with those rules so that the computer seems to "know" something about
chemistry. You can draw an organic compound and the program will try to give
it a chemical name according to the naming conventions of the International
Union of Pure and Applied Chemistry (IUPAC).
Click on the Cancel button to get out of the Lewis Dot structure mode
and return to the main program mode. To draw a compound, select the arrow tool
(upper left-most tool), click on an atom symbol, then click on the main window
area. To make a bond between two atoms, click on the bond icon (single, double
or triple), and drag the pointer between the two atoms you wish to bond. To
erase an atom, select the arrow tool, click on the atom, and pull down the
Edit menu and select Clear.
b. Draw methane (CH4). Then pull down the Struct menu and select
Find IUPAC Name. The program should print out "This is methane." Click
the OK button. Erase the methane structure by selecting Select
All and then Clear from the Edit menu.
c. Try to draw the hydrocarbons C2H6 and then C3H8 and find out what name does
the program give for them? After each structure is named, click the OK
button and erase the structure by selecting Select All and then
Clear from the Edit menu.
d. With the structure for C3H8 still on the screen, pull down the
Redraw menu and select Lewis Structure. This will cause the
programs to redraw your structure more neatly, so you can draw messily and have
the computer clean it up. Now pull down the Redraw menu and select
Line Segment. This displays the molecule in a shorthand notation that
is widely used by chemists. Can you explain how the shorthand notation works?
Hint: go back to the Lewis Structure, add another methyl group (-CH3) and see
what that looks like as a line segment drawing. How are carbon atoms
represented in line segment models?
How are hydrogen atoms connected to carbon atoms represented in line
segment models?
e. You can use this shortcut in your own drawings. For example, try using the
line (single bond) tool to draw seven line segments connected end-to-end
in a zig-zag pattern. What name does the program give for that compound?
Convert it to a Lewis structure. How many carbons does it have?
f. Try using this shortcut to draw a branched hydrocarbon, such as this
isomer of butane:
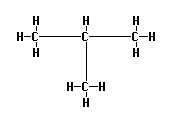
What name does Beaker give for this compound? Did you expect this name?
g. Try drawing some other linear and branched hydrocarbons of various lengths
and branching. In each case, get the program to give the IUPAC name. Write
down the structures and their names. On the basis of the evidence you
accumulate in this way, figure out how the names for such compounds are
derived. Hint: What's the longest chain of carbons you can find in each
structure?
h. Test your theory by predicting the name of some new hydrocarbon and checking
it against the name that the program gives.
3. Molecular Visualization in MacMolecule
MacMolecule is a free (public domain) molecular visualization program
developed at the University of Arizona that is used to study the
three-dimensional shapes of molecules. MacMolecule does not work
on the newer PowerMacs in the computer lab; it works only on the old Mac II
computers with color monitors; work in pairs on the machines . Open
(double-click on) the Temporaty Storage folder, open the MacMolecule folder,
and open MacMolecule. Click on the flash screen to continue.
a. Pull down the File menu and select Open. Scroll to the
Small Molecule folder, open it, and scroll to Oxygen - B&S,
and open this file. This shows O2 as a ball and stick model, very much
like the plastic model kit we used in class. Position the mouse pointer on the
model and press the mouse button, and drag slowly in various directions. What
happens?
b. Pull down the Options men and select Model. The pop-up side
menu gives three choices; select Space Filling. Scientists believe that
these space filling models give a more realistic view of the actual shape of a
molecule than the ball and stick. Can you rotate the space filling model?
c. Close the oxygen model by clicking on the "close box" in the upper left
corner of the window. Open the model for water and look at it in both
ball and stick and space filling modes. What colors are used for hydrogen and
oxygen?
d. Close the water model by clicking on the "close box" in the upper left
corner of the window. Open the models for methane and for carbon
dioxide and look at these in both ball and stick and space filling modes.
Describe how the space filling model differs in appearance from the ball and
stick model.
e. Open the model for buckminsterfullerene and, before trying to rotate
it, change to a wireframe model. Wireframe models are the least
realistic but are easier to draw and can be manipulated more quickly that the
other models, especially for large molecules such as this. Rotate the model
and note how the carbon atoms form rings or 5 and 6 carbons. How many carbon
atoms are there altogether? (View in ball-and-stick mode). Are there really holes in the center of the carbon
rings? (View in space filling mode).
f. Open the Small Inorganics folder and by opening and inspecting the
molecules in that folder, determine the colors that MacMolecule uses to
represent the various atoms. Enter the colors in the table on the next page.
Based on the molecules in this folder, what atoms does MacMolecule have that
your plastic model kit did not have?
Hydrogen
Carbon
Oxygen
Nitrogen
Fluorine
Chlorine
Bromine
Iodine
Sulfur
Phosphorous
Silicon
Xenon
g. Based on the models in the Small Inorganics folder, what do the following
prefixes mean?
di-
tri-
tetra-
penta-
hexa-
h. Which of the molecules in the Small Inorganics folder contain
nitrogen atoms?
i. Many chemistry were surprised when the xenon difluoride and xenon
tetrafluoride were discovered some years ago. Why do you think were they
surprised? (Hint: how many valence (outer shell) electrons does Xenon have,
based on its position in the periodic table?)
Go to Chem 121 Home Page
This page is maintained by Prof. T.C. O'Haver, Department of Chemistry and
Biochemistry. Comments, suggestions and questions should be directed to
Prof. O'Haver at to2@umail.umd.edu.
Created April 16, 1996.
